the company
Meletios Therapeutics’ aim is to fulfill the urgent and large unmet medical need for broad spectrum treatments against current and emerging viral infections.
This French biotech company was created in April 2020 by a team of high level scientists and experienced biotech managers.
One of our first drug candidate has successfully demonstrated immunomodulatory properties, targeting respiratory viruses. This molecule is currently preparing for human proof of concept.
Several drug candidates from Meletios portfolio are currently being tested both in vitro and in vivo and will expand our range of candidates on different RNA viruses.
Meletios Therapeutics’ goal is to become a global leader in the fight against emerging viral infections by developing innovative broad-spectrum therapeutic solutions.
STRENGTHS OF MELETIOS
THE CONTEXT
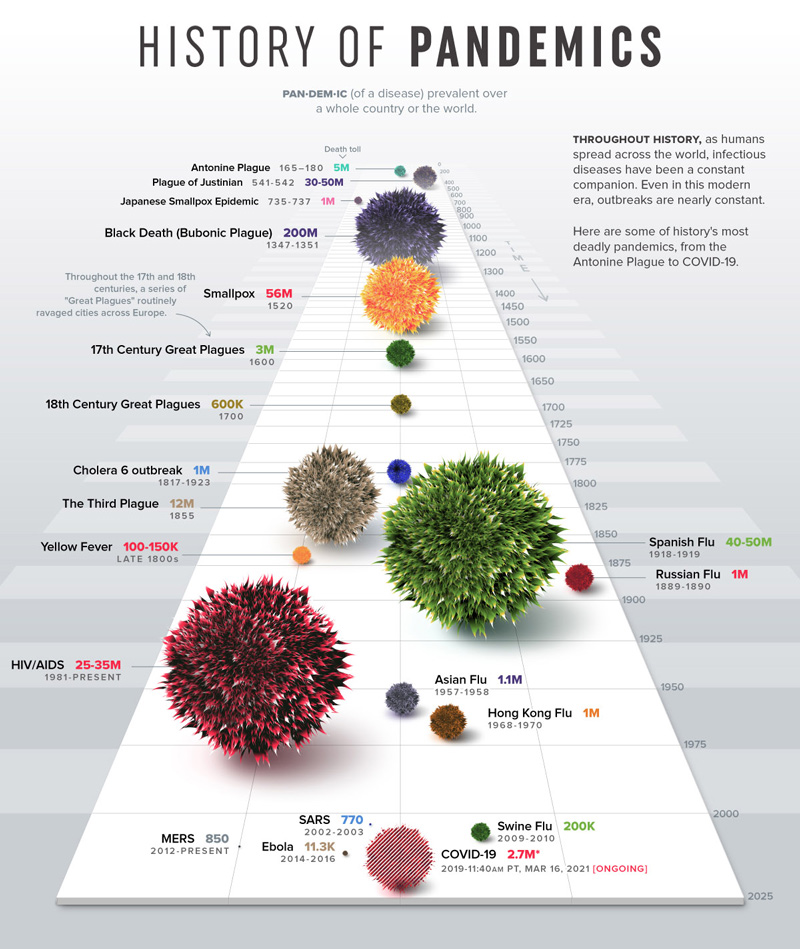
As far back as we can remember, there has always been a real concern for human communities about epidemics which have been incredibly devastating in the past. And we are nowadays seeing a speeding up of the phenomenon. We are increasingly interconnected and pushing the boundaries of our environment further and further, yielding unknown viruses to come out into the open.
There is thus a real need to anticipate the emergence of new viruses that could lead to globalized epidemics such as COVID-19.
WHY IS THERE A CRITICAL NEED FOR ANTIVIRALS?
To tackle future emerging viruses, every effort must be made to channel epidemics. Vaccines and treatments are two complementary therapeutic strategies.
Vaccines are an essential preventive tool but have the disadvantages of targeting a virus that must be already (well) known and of being uncertain about possible variants. They can be also time-consuming to implement.
An alternative is therefore needed for patients who are already ill in order to treat their symptoms and to resolve the infection from the start of the epidemic. There is a significant need for broad-spectrum therapies that have a low risk of inducing treatment resistance and can be administered throughout the course of the disease, not just in the first few days after infection.
DISEASES
We are facing an increasing number of pandemic situations
New viruses are to emerge with a lack of overlap between viruses
VACCINES
= PREVENTION
Vaccines are mostly efficient against one strain only
Implementation in the population takes long
Viral genome are small and evolve rapidly
It is sometimes impossible
to design a vaccine for a virus
ANTIVIRAL
= CURE
The current antiviral armamentarium is not sufficient to face current and future epidemics
Broad spectrum treatment could be used as soon as a viral pandemic is identified
Antivirals will be efficacious against a large panel of viruses
MELETIOS TEAM
Our Board
OUR SCIENTIFIC ADVISORS
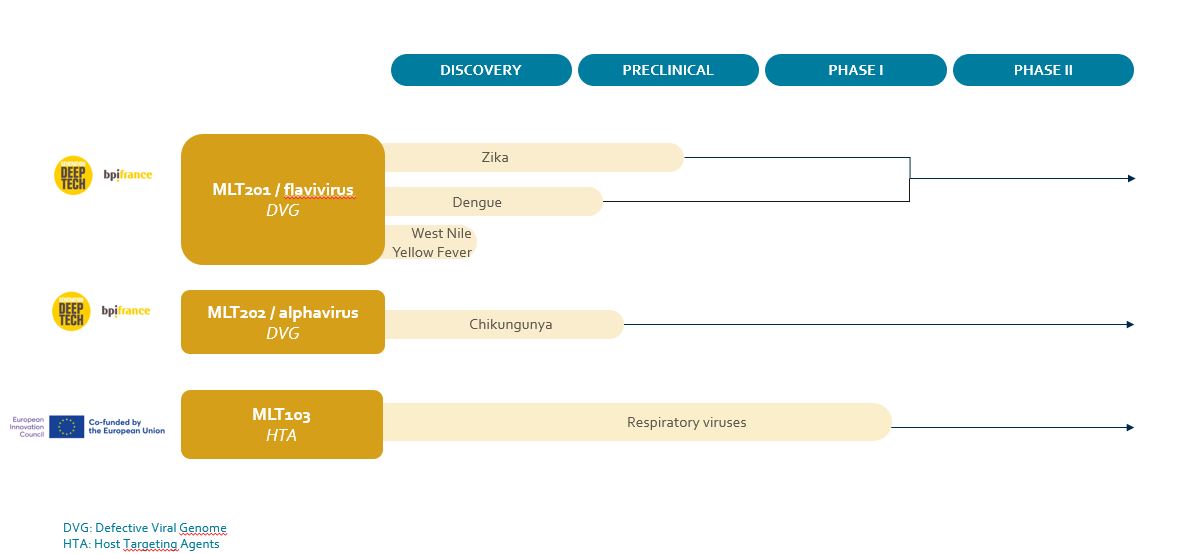
meletios’ R&D STRATEGY
Meletios develops two complementary programs to prevent the virus from taking advantage of the host resources.
Meletios is developing a disruptive platform for new RNA biologics, Defective Viral Genomes (DVG), originating from the Institut Pasteur. The RNA sequences directly interfere with the protein expression machinery hijacked by viruses. Candidates have a broad spectrum potential and are under development for arboviruses (Dengue, Zika, Chikungunya). The platform is RNA virus agnostic and is the perfect tool for pandemic preparedness with the capacity to generate a promising candidate DVG within months only.
The most advanced drug candidate is a host targeting agent that has demonstrated an immunomodulatory activity in respiratory viral diseases and acute inflammation models. MLT103 is dedicated to answer the high medical need for treatment of inflammation dysregulation in respiratory viral infections. This molecule has proved its safety in humans and next step in the human proof of efficacy.
DEFECTIVE VIRAL GENOMES
Target:
Protein expression machinery hijacked by viruses
What:
RNA sequences
Strength:
– Disruptive technology platform
– Originating from a prestigious academic center: Institut Pasteur
HOST TARGETING AGENTS
Target:
Metabolic resources hijacked by viruses
What:
Small molecules
Strength:
Biomarkers predictive of viral diseases
NEWS
2 March 2022 – License agreement with the Institut Pasteur for a new class of antivirals: Interview – Marco Vignuzzi (Institut Pasteur) x Catherine Martre (Meletios Therapeutics)
Careers
You are interested in joining our team? Do not hesitate to submit your application with a cover letter and resume at contact@meletiostx.com!
Please send your application to contact@meletiostx.com
Get in touch with us
You are interested in a partnership opportunity, a job or just want to know more about us? We’d like to hear from you. Don’t hesitate to reach us directly!